2In the first three quarters of 018, the proportion of the top ten batches of vaccine products issued in China exceeded 88%. In the following, the Prospective Industry Research Institute will interpret the first three quarters of China's vaccine industry from the perspective of subdivided products.
1. Rabies vaccine
Judging from the monthly data of the issuance of rabies vaccines in China from January to September 2018, the overall trend is fluctuating, but there was an abnormally low value in July. Prospective Industry Research Institute believes that it is mainly affected by the "Changchun Eternal Vaccine" event .
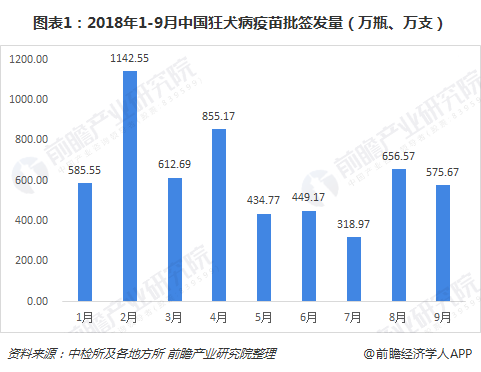
Prior to this incident (July 2018), Changchun Changsheng ranked third in the domestic rabies vaccine supplier. Even if it was issued for three consecutive months from July to September, it was 0, and the ranking in the first three quarters still ranked fourth in the country. It can be seen that its position in the domestic rabies vaccine market is precisely because of its large share in the market, this event will cause widespread panic and anger among the public.
From the breakdown of rabies vaccine products, it is currently divided into four types: freeze-dried human rabies vaccine (Vero cells), freeze-dried human rabies vaccine (human diploid cells), human rabies vaccine (Vero cells), Human rabies vaccine (hamster kidney cells), from the specific situation of the first three quarters of 2018, freeze-dried human rabies vaccine (Vero cells) is currently the mainstream product in the rabies vaccine market.
Second, hepatitis B vaccine
From January to September 2018, the total number of batches of hepatitis B vaccine issued in China was 53.290 million (bottles and sticks). From month to month, the number of batches issued in January, March and April was relatively large, at 9 million (bottles, The number of batches issued in July and August is more than 6 million (bottles and sticks). Relatively speaking, the number of batches issued in June is the least, only 592,700 (bottles and sticks).
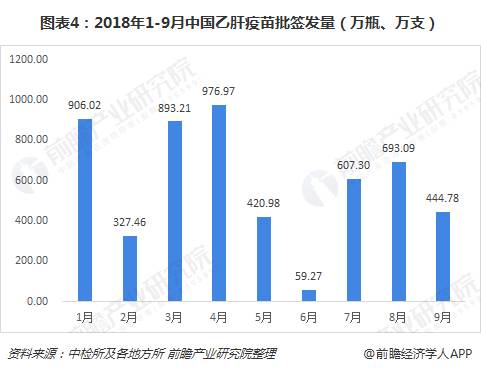
Judging from the situation of hepatitis B vaccine enterprises, there were a total of 5 manufacturers in China's hepatitis B vaccine market in the first three quarters, of which Kangtai Biotech and Hanxin Biotech had relatively large batches of issuance, accounting for 44% and 34%, respectively; 3 The companies are Jintan Biotechnology, Hualan Biotechnology and foreign-funded companies GlaxoSmithKline, accounting for 15%, 4% and 3% respectively.
From the perspective of products, the current hepatitis B vaccine is mainly divided into three types: recombinant hepatitis B vaccine (Hansen yeast), recombinant hepatitis B vaccine (Saccharomyces cerevisiae), recombinant hepatitis B vaccine (CHO cells), accounting for In comparison, the current market share of the first two products is relatively large, accounting for 38% and 47% of the first three quarters of 2018 respectively; the batch of recombinant hepatitis B vaccine (CHO cells) accounted for 15% ,Relatively small.
Three, one hundred, white, broken vaccine
From January to September 2018, China issued a total of 49.907 million (bottles and bottles) of batches of 100, white and broken vaccines. From month to month, the most was April, which was 11.3677 million (bottles and bottles). Monthly decline, the number of batches issued in September 2018 was 3.172 million (bottles and sticks).
Judging from the situation of the 100, 100, white, and broken vaccine companies in the first three quarters, there are three manufacturing companies, of which Wuhan Biotech's batch issuance accounted for 94%, occupying an absolute market advantage.
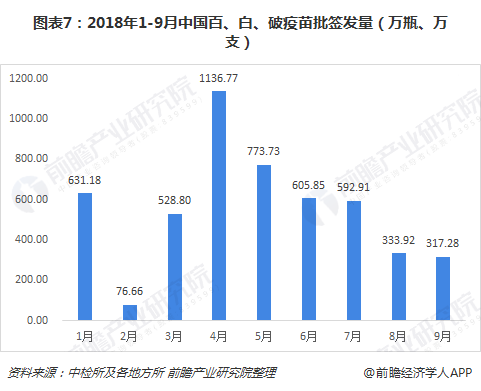
Judging from the batch issuance volume of the subdivided products, the current mainstream market is the adsorption of cell-free Baibai combined vaccine, accounting for 83% in the first three quarters of 2018.
IV. Meningitis vaccine
From January to September 2018, the total number of Chinese meningitis vaccines issued was 48.209 million (bottles and sticks). From month to month, the batches issued in January, May and August were all over 6 million (bottles and sticks) In April and September, the issuance volume was relatively small, less than 4 million (bottles and sticks).
Judging from the proportion of enterprises, in the first three quarters of 2018, the overall concentration of enterprises was relatively high, and the market share of the top three enterprises reached 90%.
From the perspective of subdivided products, Group A and Group C meningococcal polysaccharide vaccines are currently the mainstream meningitis vaccine products, accounting for 66% in the first three quarters of 2018; Group A meningococcal polysaccharide vaccines and ACYW135 group meningococcal polysaccharide vaccines Accounted for 27% and 7%, respectively.
V. Polio vaccine
From January to September 2018, the total number of polio vaccines issued in China totaled 40.7995 million (bottles and sticks). By month, the most batches were issued in May, reaching 7.7634 million (bottles and sticks), the least In July, it was 2.5597 million (bottles and sticks). Overall, the monthly batch of polio vaccine is relatively stable.
From the perspective of enterprises, there are mainly three polio vaccine manufacturers in China in the first three quarters of 2018, of which the number of batches issued by Beishengyan Biology is 66%, which has obvious advantages.
Six, JE vaccine
From January to September 2018, the total number of JE vaccines issued in China totaled 40.122 million (bottles and sticks). From month to month, the overall trend of fluctuations was the highest. In March 2018, the most was 627.93 million (bottles and sticks) In May, June, and September, the batch issuance volume also exceeded 5 million (bottles and sticks).
From the point of view of enterprises, there are currently three JE vaccine production enterprises in China, and the concentration of enterprises is relatively high. The proportion of batches issued by Chengdu Biology and Wuhan Biology is 61% and 33%, respectively.
From the perspective of subdivided products, live attenuated Japanese encephalitis vaccine is currently the mainstream product in the market, accounting for 93.86% in the first three quarters of 2018; the approval of freeze-dried inactivated Japanese encephalitis vaccine and inactivated Japanese encephalitis vaccine The proportion of issued volume was 5.95% and 0.19% respectively.
Seven, hemp, gill, wind vaccine
From January to September 2018, the total number of issued Chinese measles vaccines was 29.4942 million (bottles and sticks). From month to month, the most was March, which was 4.202 million (bottles and sticks), and the least was 2 It was only 84,100 (bottles and sticks) per month.
From the perspective of enterprises, there are five major manufacturers of hemp, gill and wind vaccines in the first three quarters of 2018. Among them, the number of batches issued by Beishengyan Biological and Shanghai Biological are 38% and 39%, respectively. The mainstream manufacturers in the wind vaccine market.
In terms of products, there are currently 4 types of subdivided products in the measles, gills and wind vaccine market, of which the most is the live attenuated combined vaccine against measles, accounting for 55%; the second is the combined measles and rubella vaccine, accounting for 24 %, The proportion of measles mumps combined live attenuated vaccine and mumps live attenuated vaccine is 5% and 16% respectively






