1. Classification and sales analysis of influenza vaccine
1. Classification of influenza viruses and vaccines
Influenza is short for influenza, and refers to diseases of the respiratory tract and other organs caused by influenza viruses. Every year there will be varying degrees of prevalence in winter and spring. Human influenza viruses are divided into three types (A), B (B), and C (C), which are the pathogens of influenza. Influenza A virus is the most susceptible to mutations and can infect humans and a variety of animals. It is the main pathogen of human influenza, and H1N1 and H3N2 have been prevalent in the population for a long time. For example, the 2009 global pandemic of H1N1 influenza caused at least 12,000 deaths. Influenza B virus generally does not cause pandemic worldwide, but it can cause regional and seasonal epidemics. Influenza C viruses can only cause mild upper respiratory tract infections in humans and rarely cause epidemics. Therefore, the current influenza vaccine is mainly for the prevention of seasonal influenza A (H1N1) and A (H3N2), and influenza B.
At present, the most effective way to prevent the pathogenicity and epidemic of human influenza is still vaccination. There are three main types of influenza vaccines: the first-generation whole virus inactivated vaccine, the second-generation split vaccine and the third-generation subunit vaccine, all of which belong to the second-class vaccine. Each vaccine contains three influenza inactivated viruses or antigen components of subtypes A1, A3, and B. At present, the main issue of the China Inspection Institute is the split influenza vaccine. This vaccine splits the influenza virus on the basis of the inactivated vaccine, which has good immune effect and high safety.
2. Flu vaccine sales bottomed out
Influenza vaccination has a strong seasonality, generally starting from July to August every year, until the beginning of the second spring. Affected by the outbreak of pandemic influenza in 2009-2010, the number of domestic influenza vaccine batches reached a peak of more than 60 million, and then began to fall and stabilized at 30-40 million. In 2017, more than 26 million national influenza vaccines were issued. Compared with 2016, it has increased by about 4 million, a year-on-year increase of nearly 20%; it is estimated that more than 20 million people have actually been vaccinated. Based on 50 yuan per person, the terminal market scale exceeds 1 billion yuan. In 2017, with the outbreak of the epidemic and the increase in people's awareness of vaccination, influenza vaccines are likely to bottom out.
At present, the main domestic market is the trivalent influenza vaccine (including two types of influenza A and one type B influenza), and the quadrivalent influenza has not yet been listed. There are 15 domestic manufacturers that have obtained qualifications for the production of influenza split vaccines. As market competition intensifies, some manufacturers are forced to withdraw from the market. Only 5-6 companies have issued data in the past two years.
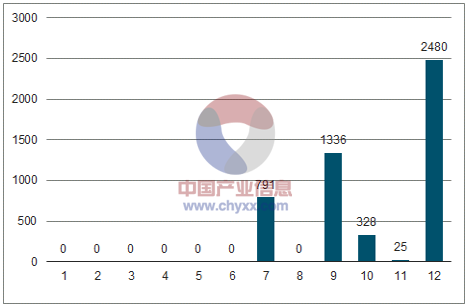
Number of batches of influenza vaccines approved by month in 2017 (ten thousand)
2. Influenza vaccine is a veritable blockbuster variety with important promotion value
Although WHO has repeatedly pointed out that vaccination against influenza is the best way to prevent influenza, compared with the United States, there are only more than 20 million people vaccinated against influenza every year in China, and the vaccination rate is less than 2%, which is only 1/20 of the United States. The data shows that in 2017, the vaccination rate of the entire population was 38.6%, of which 38.8% were children and adolescents, and 38.5% for adults. In 2020, the vaccination rate of the entire population is planned to reach 70%. There are currently 115 countries in the world that have included influenza vaccines in their national immunization plans, including some low- and middle-income countries and countries that have included influenza vaccines in their national immunization plans, including some low- and middle-income countries and one low-income country. In contrast, with the gradual improvement of domestic economic conditions and the gradual increase in medical and health investment, there is still huge room for improvement in the domestic influenza vaccination rate.
The United States CDC recently based on a robust multi-country survey showing that 291,000 to 646,000 people die from respiratory flu-related respiratory diseases each year worldwide, which is higher than the previous estimate of 250,000 to 500,000 people; 12,000 to 56,000 people die from influenza in the United States each year . However, data from the Ministry of Health of China shows that in 2017, the number of influenza deaths in China was only 41, and even in the January-February 2018 period, there were only 106 people, less than 1% of the United States. Therefore, Chinese people generally have misunderstandings about influenza, that is, Chinese people are immune to influenza, and usually do not die from influenza.

