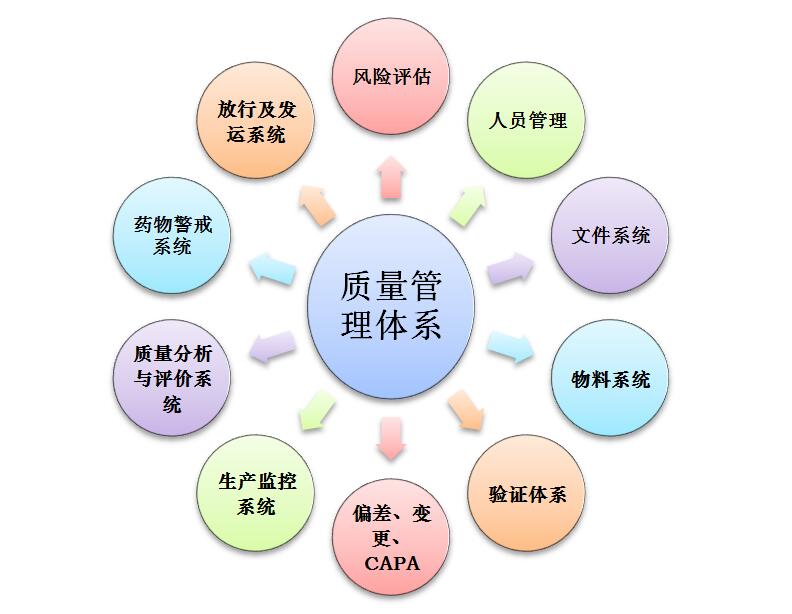
Our company's quality management system is established on the basis of referring to the current GMP of FDA, EU and WHO and complying with the requirements of China's new GMP. The system uses risk assessment methods, and the quality management system covers the entire life cycle of the product, including product development, technology transfer, commercial production, and product delisting. Established a three-level quality management document system and perfect quality management procedures such as deviation investigation, change control, GMP training, verification, cold chain transportation, complaint handling, recall, annual review, and adverse reaction monitoring, etc. Effective and stable.



